How do we know how old something is? In the case of people, we ask for a birth certificate. For trees, we count tree rings- Dendrochronology. But how do we know how old a fossil is?
Carbon dating is a form of radiometric dating. Carbon-14 dating was invented by Willard Libby and won a noble prize in chemistry in 1960 for this. It is one of the most valuable tools for dating fossils and involves analyzing the ratio of the two types of carbon atoms. This helped us understand what the world was like thousands of years ago.
The age of fossils can be known by looking at the ratio of the two types of carbon atoms present in fossils. Every living thing is made of carbon. Plants obtain carbon dioxide from the atmosphere and form complex molecules. Animals get their carbon by eating these plants. Humans eat animals and that’s how carbon is found everywhere.
How does Carbon-14 dating work?
Carbon-14 is formed when the sun’s rays interact with stable atmospheric nitrogen. There are two forms of carbon in the atmosphere – carbon-12 (C12) and carbon-14 (C14). C12 is very abundant and stable whereas C14 on the other hand is unstable. So once an animal dies, C14 begins to decompose in its body.
If their sample has less carbon-14 than normal, it means it has been around long enough for the carbon to decay, so it is old. But if they have a sample where the ratio is normal, it’s probably relatively new.
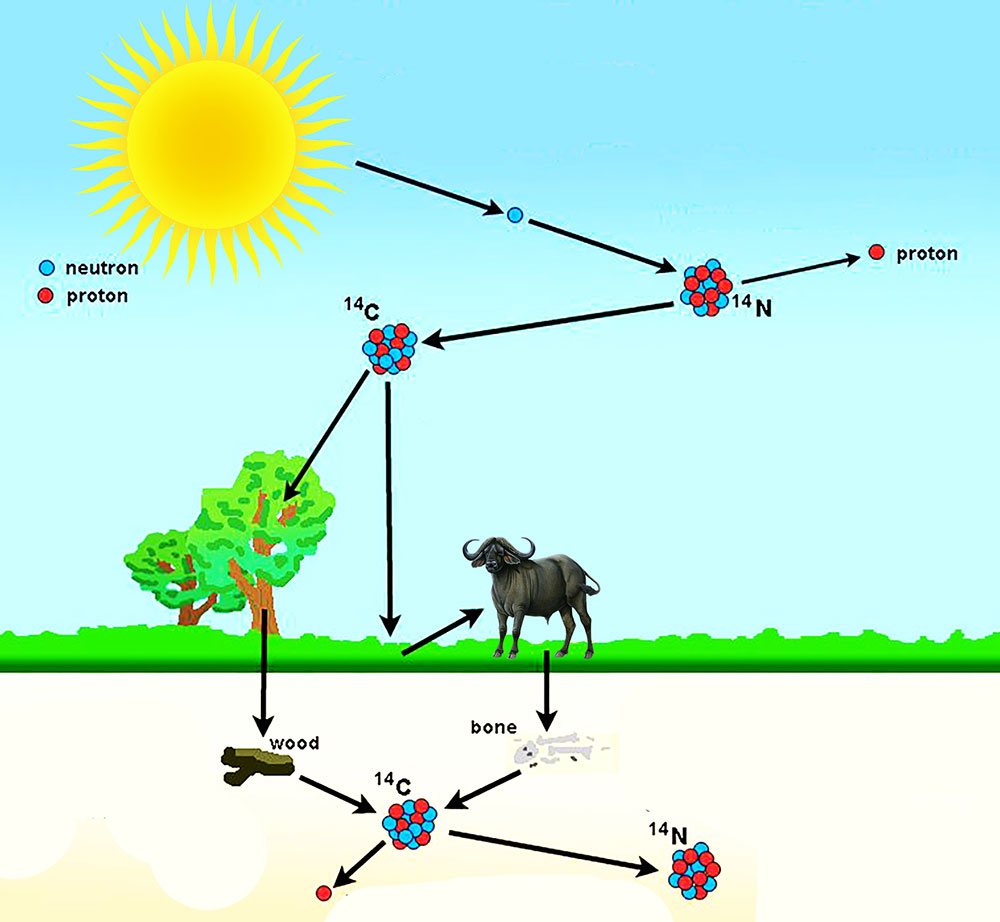
On average, every 5,730 years, about half of the C14 atoms will decay into nitrogen. This is its half-life. Half-life is the amount of time it takes for the number of radioactive particles in a sample to be reduced by half. After one half-life i.e. 5730 years, the animal that is fossilized will have about half the amount of C14 it started with. After another half-life i.e. 11,460 years, it will have about a quarter of what was originally present. And after another half-life, i.e. 17,190 years, it will have about an eighth of the number of carbon-14. And so on, and so on, until there is none left. On the contrary, the amount of C12 in its body will remain the same.
By measuring the ratio of C14 to C12, we can measure how many thousand years have passed since the animal died. The way this is done is by measuring the rate of decay of carbon-14 atoms in comparison to the slow and stable carbon-12 atoms that are there.
Limitation of carbon-14 dating
- Carbon dating works for fossils up to about 50,000 years old. For older fossils, scientists use unstable elements that have much longer half-lives.
- This is useful for dating organic material. This does not work for inorganic materials like rocks as there is no carbon content present in them.
- The chances of contamination of the samples are high.
- Carbon dating is expensive and sometimes unreliable.
- The ratio of carbon-14 to carbon-12 is not constant because of changes happening in environmental activities.
Conclusion

Because of us humans, future archaeologists are going to have a lot of trouble using carbon -14 dating. Our industrial activities are adding more and more carbon dioxide to the atmosphere which is disturbing the ratio of carbon-12 to carbon-14. Even all nuclear bombs also messing with the atmosphere.
References
Radiocarbon helps date ancient objects—but it’s not perfect